Last Updated on April 29, 2025 by XAM CONTENT
Hello students, we are providing case study questions for class 8 science. Case study questions are the new question format that is introduced in CBSE board. The resources for case study questions are very less. So, to help students we have created chapterwise case study questions for class 8 science. In this article, you will find case study questions for cbse class 8 science chapter 11 Chemical Effects of Electric Current.
| Chapter | Chemical Effects of Electric Current |
| Type of Questions | Case Study Questions |
| Nature of Questions | Competency Based Questions |
| Board | CBSE |
| Class | 8 |
| Subject | Science |
| Useful for | Class 8 Studying Students |
| Answers provided | Yes |
| Difficulty level | Mentioned |
| Important Link | Class 8 Science Chapterwise Case Study |
Case Study Questions on Chemical Effects of Electric Current
Questions
Question 1:
Read the given passage below and answer the question:
Electrolysis is defined as a process of decomposing ionic compounds into their elements by passing a direct electric current through the compound in a fluid form. The cations are reduced at cathode and anions are oxidised at the anode. The main components that are required for conducting electrolysis are an electrolyte, electrodes, and some form of external power source is also needed. Electrolysis is usually done in a vessel named ‘electrolytic cell’ containing two electrodes (cathode and anode) connected to a direct current source and an electrolyte which is an ionic compound undergoing decomposition. In the process of electrolysis, there is an interchange of ions and atoms due to the addition or removal of electrons from the external circuit. Basically, on passing current, cations move to the cathode, take electrons from the cathode (given by the supply source-battery), and is discharged into the neutral atom. The neutral atom, if solid, is deposited on the cathode and if gas, move upwards. This is a reduction process and the cation is, reduced at the cathode. At the same time anions, give up their extra electrons to the anode and is oxidised to neutral atoms at the anode. Electrons released by the anions travel across the electrical circuit and reach the cathode completing the circuit. Electrolysis involves a simultaneous oxidation reaction at anode and a reduction reaction at the cathode.
Q. 1. Which most commonly used liquid is decomposed by the process of electrolysis?
(a) Water
(b) Petrol
(c) Diesel
(d) Milk
Difficulty Level: Easy
Ans. Option (a) is correct.
Explanation: The decomposition of waterby passing electric current through it is called hydrolysis in which dissociated hydrogen and oxygen appear in form of gas bubbles on the electrode,
Q. 2. Which of the following process is based on the principles of electrolysis?
(a) Rusting
(b) Colour change of electrolyte
(c) Electroplating
(d) None of the above
Difficulty Level: Easy
Ans. Option (c) is correct.
Electroplating is the process of depositing a layer of any desired metal on another material by means of electricity in an electrolytic cell. Metal ions in the electrolyte decompose on passing electric current and get deposited on cathode forming a thin layer on the metal object.
Q. 3. ____________ is a compound which in aqueous solution that allows an electric current to pass through it while __________are the metal rods which are dipped in electrolyte and attached to external power source.
(a) Electrode, electrolyte
(b) Anode, cathode
(c) Solution, electric plate
(d) Electrolyte, electrode
Difficulty Level: Easy
Ans. Option (d)
Explanation: Electrolysis is usually done in a vessel named ‘electrolytic cell’ containing two electrodes (cathode and anode) connected to a direct current source and an electrolyte which is an ionic compound undergoing decomposition.
Q. 4. Draw an electrolytic cell.
Difficulty Level: Medium
Ans.
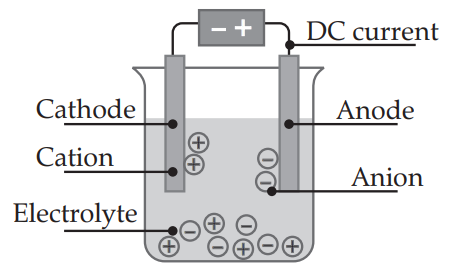


Also check
- Light Class 8 Case Study Questions Science Chapter 13
- Some Natural Phenomena Class 8 Case Study Questions Science Chapter 12
- Chemical Effects of Electric Current Class 8 Case Study Questions Science Chapter 11
- Sound Class 8 Case Study Questions Science Chapter 10
- Friction Class 8 Case Study Questions Science Chapter 9
- Force and Pressure Class 8 Case Study Questions Science Chapter 8
- Reaching the Age of Adolescence Class 8 Case Study Questions Science Chapter 7
- Reproduction in Animals Class 8 Case Study Questions Science Chapter 6
- Conservation of Plants and Animals Class 8 Case Study Questions Science Chapter 5
- Combustion and Flame Class 8 Case Study Questions Science Chapter 4
- Coal and Petroleum Class 8 Case Study Questions Science Chapter 3
- Microorganisms: Friend and Foe Class 8 Case Study Questions Science Chapter 2
- Crop Production and Management Class 8 Case Study Questions Science Chapter 1
🚀 Boost Your Exam Prep: Get case study questions for all subjects (Class 6-12) now!
👉 Explore more resources on CBSE Class 8
You may also like
Read more
- Assertion Reason Questions for Class 8 Social Science
- Assertion Reason Questions for Class 8 Maths
- Case Study Questions for Class 8 Social Science
- Case Study Questions for Class 8 Maths
Topics from which case study questions may be asked
- Understand the conduction of electricity in liquids.
- Learn about the chemical effects of electric current.
- Know about the process of electrolysis.
- Learn electroplating and its uses.
Some liquids are good conductors of electricity and some are poor conductors. Most liquids that conduct electricity are solutions of acids, bases and salts.
Air is a poor conductor of electricity. But during thunderstorms air acts as conductor and electric current pass through it to reach the Earth. This happens due to a phenomenon termed as Dielectric Breakdown.
Helpful Links for CBSE Class 8 Science Preparation
Frequently Asked Questions (FAQs) on Chemical Effects of Electric Current Case Study Questions
Q1: What are case study questions for CBSE examinations?
A1: Case study questions in CBSE examinations typically involve scenarios or real-life examples, requiring students to apply their understanding of concepts to solve problems or analyze situations.
Q2: Why are case study questions important for understanding class 8 science chapters?
A2: Case study questions provide a practical context for students to apply theoretical knowledge to real-world situations, fostering deeper understanding and critical thinking skills.
Q3: How should students approach answering case study questions for CBSE?
A3: Students should carefully read the case study, identify the key issues or problems presented, analyze the information provided, apply relevant concepts and principles of sound, and formulate well-supported solutions or responses.
Q4: Are there any resources available online for students to practice case study questions on class 8 science chapters for CBSE exams?
A4: Yes, several educational websites offer case study questions for CBSE students preparing for science examinations. We also offer a collection of case study questions for all classes and subject on our website. Visit our website to access these questions and enhance your learning experience.
Q5: How can students effectively prepare for case study questions on Chemical Effects of Electric Current for CBSE exams?
A5: Effective preparation strategies include regular revision of concepts, solving practice questions, analyzing case studies from previous exams, seeking clarification on doubts, and consulting with teachers or peers for guidance and support.
Q6: How can teachers incorporate case study questions on Chemical Effects of Electric Current class 8 science into classroom teaching?
A6: Teachers can integrate case studies into lesson plans, group discussions, or interactive activities to engage students in active learning, promote problem-solving skills, and facilitate a deeper understanding of concepts of sound.
Q7: Why sometimes the bulb in the electric tester in a complete circuit does not glow even if liquid is a good conductor of electricity?
A7: In some situations, even though the liquid is conducting, the bulb may not glow. The bulb in a circuit light up due to the heating effect of electric current.
If the current is weak, the filament does not get heated and it does not glow. The current is weak because though a material may conduct electricity,
it may not conduct it as easily as a metal
Q8: Distilled water is a bad conductor of electricity. Why does rainwater conduct electricity?
A8: Rainwater contains dissolved salts which conduct electricity. That is why distilled water is a bad conductor of electricity, but rainwater conducts electricity.
Q9: Will an electric device work if we place the positive terminal of a battery towards the negative point of the device? Explain your answer
A9: The electric device will not work as there will be insufficient current flow from the battery to the device.
Q10: Give the full form of LED. Why it is preferable over bulb in testing electric passage through a circuit?
A10: LED is (Light Emitting Diodes), It is preferable than electric bulb because LED glows even if a weak electric current flows through it.



