Last Updated on December 17, 2025 by XAM CONTENT
Hello students, we are providing case study questions for class Class 8 Science. Case study questions are the new question format that is introduced in CBSE board. The resources for case study questions are very less. So, to help students we have created chapterwise case study questions for class Class 8 Science. In this article, you will find case study questions for cbse class Class 8 Science chapter 8 Nature of Matter: Elements, Compounds, and Mixtures.
| Chapter | Nature of Matter: Elements, Compounds, and Mixtures |
| Type of Questions | Case Study Questions |
| Nature of Questions | Competency Based Questions |
| Board | CBSE |
| Class | Class 8 |
| Subject | Maths |
| Useful for | Class 8 Studying Students |
| Answers provided | Yes |
| Difficulty level | Mentioned |
| Important Link | CClass 8 Science Chapterwise Case Study |
Case Study Questions on Nature of Matter: Elements, Compounds, and Mixtures
Case Study 1: Electrolysis and the Composition of Water (Based on Activity 8.3)
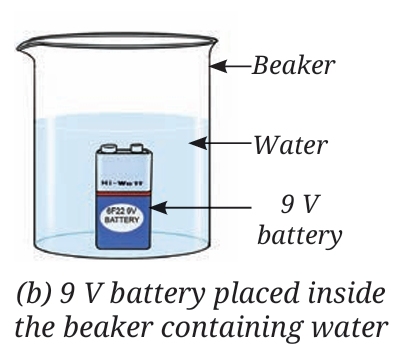
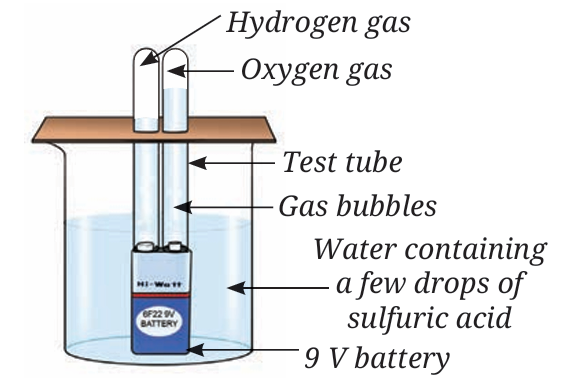
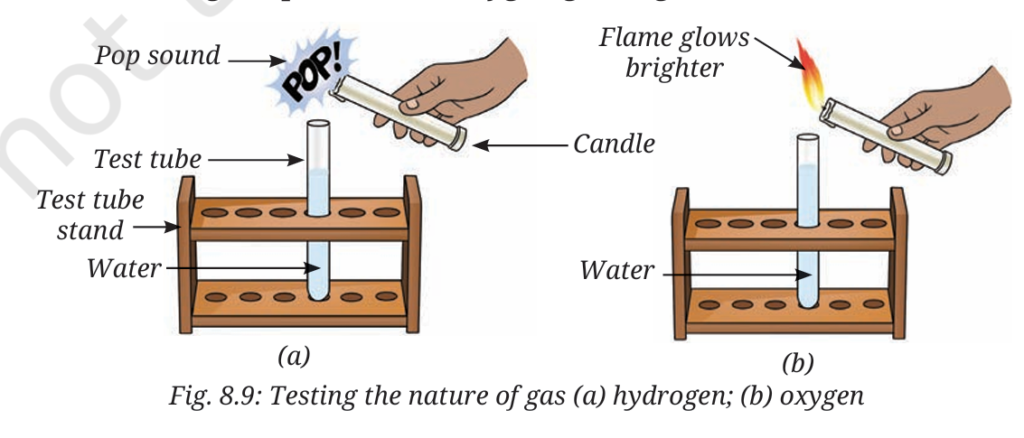
A chemistry teacher sets up a demonstration (Refer Activity 8.3) to investigate the nature of water. A beaker is filled with water containing a few drops of dilute sulfuric acid. Two water-filled test tubes are inverted over the terminals of a 9 V battery placed inside the beaker. After allowing the process to continue for 15 minutes, gas bubbles are observed collecting inside both inverted test tubes. The gas volume in one tube is noticeably double that of the other. The gas with the larger volume is removed and tested with a burning candle, resulting in a distinct “pop” sound.
Questions
Q1. The primary conclusion drawn from the difference in the volume of gases collected and the appearance of two distinct gases is that water:
A. Is a mixture because physical means (electricity) separate its components.
B. Is an element because its particles are identical (atoms).
C. Is a compound because it is composed of two elements chemically combined in a fixed ratio.
D. Is a uniform mixture because the components cannot be distinguished.
Q2. Explain the observed result that the gas volume in one test tube is double the volume in the other.
Q3. Why are the collected gases guaranteed not to be water vapour, even though they originated from water?
Q4. The gas that causes the burning candle flame to glow brighter is produced from the decomposition. Based on the 2:1 ratio, what is the chemical role of this gas in comparison to the gas that burns with a “pop” sound?
Answers
A1. C. Is a compound because it is composed of two elements chemically combined in a fixed ratio.
Explanation: The experiment shows that passing electricity through water breaks it down into two simpler substances, hydrogen and oxygen, proving water is not an element. Since these two elements are separated from water, which originally contained them, and are known to be combined chemically in a fixed ratio (2:1), water is correctly classified as a compound.
A2. Explanation: Water (H2O) is a compound where the constituent elements, hydrogen (H) and oxygen (O), are chemically combined in a fixed ratio. The ratio of the number of atoms of hydrogen to oxygen in water is 2:1. When water decomposes into hydrogen gas and oxygen gas, the volume of hydrogen produced is double the volume of oxygen produced, resulting in the observed volume difference.
A3. Explanation: The gases collected are not water vapour because they are tested and identified as hydrogen and oxygen gases, which are the constituent elements of water. If the collected gases were simply water vapour, they would have condensed back to form liquid water, which they did not do.
A4. The gas that glows brighter is Oxygen, which supports combustion.
Step-by-step Calculation/Explanation: The experiment results state that one gas burns with a ‘pop’ sound (Hydrogen), and the other gas causes the flame to glow brighter, indicating the presence of Oxygen gas. Since the ratio of H:O is 2:1, and Hydrogen has the larger volume (pop sound, 2 parts), Oxygen must have the smaller volume (1 part). Hydrogen is classified as a fuel, while Oxygen’s role is confirmed as the element that supports combustion (“flame… will glow brighter”).
We hope the given case study questions for Nature of Matter: Elements, Compounds, and Mixtures Class Class 8 helps you in your learning.
Also check
- Curiosity Class 8 Science Chapter 13 Our Home: Earth, a Unique Life Sustaining Planet Case Study Questions
- Curiosity Class 8 Science Chapter 12 How Nature Works in Harmony Case Study Questions
- Curiosity Class 8 Science Chapter 11 Keeping Time with the Skies Case Study Questions
- Curiosity Class 8 Science Chapter 10 Light: Mirrors and Lenses Case Study Questions
- Curiosity Class 8 Science Chapter 9 The Amazing World of Solutes, Solvents, and Solutions Case Study Questions
- Curiosity Class 8 Science Chapter 8 Nature of Matter: Elements, Compounds, and Mixtures Case Study Questions
- Curiosity Class 8 Science Chapter 7 Particulate Nature of Matter Case Study Questions
- Curiosity Class 8 Science Chapter 6 Pressure, Winds, Storms, and Cyclones Case Study Questions
- Curiosity Class 8 Science Chapter 5 Exploring Forces Case Study Questions
- Curiosity Class 8 Science Chapter 4 Electricity: Magnetic and Heating Effects Case Study Questions
- Curiosity Class 8 Science Chapter 3 Health: The Ultimate Treasure Case Study Questions
- Curiosity Class 8 Science Chapter 2 The Invisible Living World Case Study Questions
- Curiosity Class 8 Science Chapter 1 Exploring the Investigative World of Science Case Study Questions
🚀 Boost Your Exam Prep: Get case study questions for all subjects (Class 6-12) now!
👉 Explore more resources on CBSE Class 8
Topics from which case study questions may be asked
- Classification of matter
- Pure substances vs. mixtures
- Elements and their symbols
- Compounds and their properties
- Types of mixtures
- Separation techniques
- Examples from daily life
All substances around us are made of elements or combinations forming compounds and mixtures.
Frequently Asked Questions (FAQs) on Nature of Matter: Elements, Compounds, and Mixtures Case Study Questions



