Last Updated on December 17, 2025 by XAM CONTENT
Hello students, we are providing case study questions for class Class 8 Science. Case study questions are the new question format that is introduced in CBSE board. The resources for case study questions are very less. So, to help students we have created chapterwise case study questions for class Class 8 Science. In this article, you will find case study questions for cbse class Class 8 Science chapter 7 Particulate Nature of Matter.
| Chapter | Particulate Nature of Matter |
| Type of Questions | Case Study Questions |
| Nature of Questions | Competency Based Questions |
| Board | CBSE |
| Class | Class 8 |
| Subject | Science |
| Useful for | Class 8 Studying Students |
| Answers provided | Yes |
| Difficulty level | Mentioned |
| Important Link | Class 8 Science Chapterwise Case Study |
Case Study Questions on Particulate Nature of Matter
Case Study 1: The Principle of Interparticle Space (Based on Activity 7.7)
A student conducts Activity 7.7 (Refer NCERT) to investigate interparticle spaces in liquids. A glass vessel is filled halfway with water (Level A). Two teaspoons of granulated sugar are carefully added, causing the level to momentarily rise to B. After thorough stirring, the sugar dissolves completely, and the final level is marked C. The student notes that the volume represented by Level C is less than the theoretical sum of the separate volumes of the water and the added sugar. The experiment is then repeated using two teaspoons of fine sand instead of sugar. The sand settles at the bottom, and the final water level is observed to be noticeably higher than the initial Level A, showing no sign of dissolution.
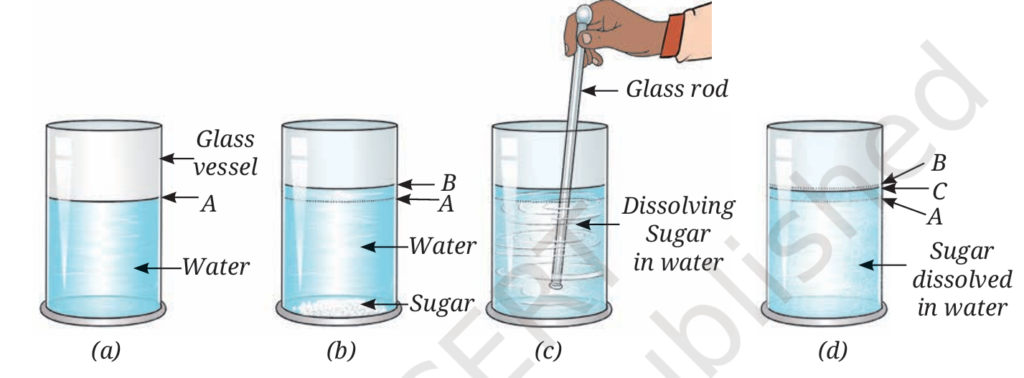
Questions
Q1. What is the main reason that the final volume of the water-sugar solution (Level C) is less than the expected volume (Level B)?
A. The sugar undergoes a chemical change, reducing its mass.
B. Water molecules react with sugar particles, changing their state.
C. The constituent particles of sugar occupy the interparticle spaces of water.
D. Water evaporates rapidly due to stirring, reducing the overall volume.
Q2. Justify the statement: “Dissolving sugar in water is a demonstration that matter is particulate in nature.”
Q3. If Vwater is the volume of water and Vsand is the volume occupied by the sand grains, express the approximate relationship between the final volume (Vfinal) of the water-sand mixture and Vwater and Vsand.
Q4. Why does sand, a solid, fail to demonstrate the phenomenon observed with sugar, another solid, when mixed with water?.
Answers
A1. C. The constituent particles of sugar occupy the interparticle spaces of water.
Reasoning: When sugar dissolves, it breaks up into its constituent particles. These tiny particles separate and distribute themselves by occupying the available spaces between the water particles, known as interparticle spaces. This mixing at the microscopic level means the final volume is not a simple sum of the initial volumes.
A2. Explanation: The successful dissolution of sugar into water, where the sugar particles seem to vanish yet their presence is confirmed by taste, supports the idea that matter is composed of extremely small particles. Crucially, the observation that the particles of sugar separate and fill the volume between the water particles demonstrates that water particles must have spaces between them (interparticle spaces), a key characteristic of the particulate nature of matter.
A3. Explanation: Vfinal≈Vwater+Vsand.
Sand is an insoluble solid in water. Since sand does not dissolve, its constituent particles cannot break up and occupy the voids between the water particles. Instead, the sand particles settle down and occupy physical space in the container, meaning the final volume of the mixture is approximately the sum of the volumes of the two components.
4. Explanation: The ability of sugar to dissolve (and hence spread into the interparticle space) depends on the forces holding its particles together. In the case of sugar, the water particles are able to pull out the constituent particles, allowing dissolution. However, in the case of sand, the constituent particles are held together so strongly that the water particles are unable to pull them out, classifying sand as insoluble in water.
We hope the given case study questions for Particulate Nature of Matter Class Class 8 helps you in your learning.
Also check
- Curiosity Class 8 Science Chapter 13 Our Home: Earth, a Unique Life Sustaining Planet Case Study Questions
- Curiosity Class 8 Science Chapter 12 How Nature Works in Harmony Case Study Questions
- Curiosity Class 8 Science Chapter 11 Keeping Time with the Skies Case Study Questions
- Curiosity Class 8 Science Chapter 10 Light: Mirrors and Lenses Case Study Questions
- Curiosity Class 8 Science Chapter 9 The Amazing World of Solutes, Solvents, and Solutions Case Study Questions
- Curiosity Class 8 Science Chapter 8 Nature of Matter: Elements, Compounds, and Mixtures Case Study Questions
- Curiosity Class 8 Science Chapter 7 Particulate Nature of Matter Case Study Questions
- Curiosity Class 8 Science Chapter 6 Pressure, Winds, Storms, and Cyclones Case Study Questions
- Curiosity Class 8 Science Chapter 5 Exploring Forces Case Study Questions
- Curiosity Class 8 Science Chapter 4 Electricity: Magnetic and Heating Effects Case Study Questions
- Curiosity Class 8 Science Chapter 3 Health: The Ultimate Treasure Case Study Questions
- Curiosity Class 8 Science Chapter 2 The Invisible Living World Case Study Questions
- Curiosity Class 8 Science Chapter 1 Exploring the Investigative World of Science Case Study Questions
🚀 Boost Your Exam Prep: Get case study questions for all subjects (Class 6-12) now!
👉 Explore more resources on CBSE Class 8
Topics from which case study questions may be asked
- Matter and its characteristics
- States of matter
- Particle theory of matter
- Interparticle spaces and forces
- Diffusion and Brownian motion
- Change of state
- Real-life applications
Matter is made up of tiny particles that are in constant motion, influencing its state and properties.
Frequently Asked Questions (FAQs) on Particulate Nature of Matter Case Study Questions



