Last Updated on May 15, 2025 by XAM CONTENT
Hello students, we are providing case study questions for class 9 science. Case study questions are the new question format that is introduced in CBSE board. The resources for case study questions are very less. So, to help students we have created chapterwise case study questions for class 9 science. In this article, you will find case study questions for cbse class 9 science chapter 2 Is Matter Around Us Pure.
| Chapter | Is Matter Around Us Pure |
| Type of Questions | Case Study Questions |
| Nature of Questions | Competency Based Questions |
| Board | CBSE |
| Class | 9 |
| Subject | Science |
| Useful for | Class 9 Studying Students |
| Answers provided | Yes |
| Difficulty level | Mentioned |
| Important Link | Class 9 Science Chapterwise Case Study |
Case Study Questions on Is Matter Around Us Pure
Questions
Question 1:
A group of students took an old shoe box and covered it with a black paper from all sides. They fixed a source of light (a torch) at one end of the box by making a hole in it and made another hole on the other side to view the light. They placed a milk sample contained in a tumbler in the box as shown in the figure below. They were amazed to see that milk taken in the tumbler was illuminated. They tried the same activity by taking a salt solution but found that light simply passed through it.
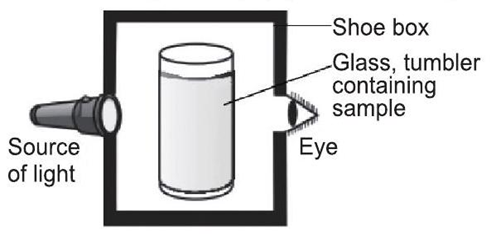
Read the given passage carefully and give the answer of the following questions:
Q 1. Explain why the milk sample was illuminated? Name the phenomenon involved.
Q2. Same results were not observed with a salt solution. Explain.
Q 3. Can you suggest two more solutions which would show the same effect as shown by the milk solution?
Q 4. Give one example of above phenomenon observed in our surroundings.
Answers
- Because milk is a colloidal solution and would show Tyndall effect.
- Salt solution is a true solution and would not scatter light.
- Soap solution and ink solution.
- Tyndall effect is observed when sunlight passes through the canopy of a dense forest.

Also check
Case study questions for other chapters of class 9 science is given below.
- Structure of the Atom Class 9 Case Study Questions Science Chapter 4
- Atoms and Molecules Class 9 Case Study Questions Science Chapter 3
- Improvement in Food Resources Class 9 Case Study Questions Science Chapter 12
- Sound Class 9 Case Study Questions Science Chapter 11
- Work and Energy Class 9 Case Study Questions Science Chapter 10
- Gravitation Class 9 Case Study Questions Science Chapter 9
- Force and Laws of Motion Class 9 Case Study Questions Science Chapter 8
- Motion Class 9 Case Study Questions Science Chapter 7
- Tissues Class 9 Case Study Questions Science Chapter 6
- The Fundamental Unit of Life Class 9 Case Study Questions Science Chapter 5
- Is Matter Around Us Pure Class 9 Case Study Questions Science Chapter 2
- Matter in Our Surroundings Class 9 Case Study Questions Science Chapter 1
We hope the given case study questions for Is Matter Around Us Pure Class 9 helps you in your learning.
🚀 Boost Your Exam Prep: Get case study questions for all subjects (Class 6-12) now!
👉 Explore more resources on CBSE Class 9
Helpful Links for CBSE Class 9 Science Preparation
- Download Latest Sample Papers for CBSE Class 9 Science
- Download Worksheets for CBSE Class 9 Science
- Download Chapter Tests for CBSE Class 9 Science
- Download Case Study Question Bank for CBSE Class 9 Science
- Download Numerical Problems for CBSE Class 9 Physics
- Download Important MCQs for CBSE Class 9 Physics
Topics from which case study questions may be asked
- Mixtures
- Separation Techniques
- Concentration of Solutions
- Physical and Chemical Change
- Experiments and Observations
This chapter deals with the basic understanding of mixtures, solutions, separation techniques, physical change and chemical change. case study questions based on above topics may be asked.
Frequently Asked Questions (FAQs) on Is Matter Around Us Pure Case Study Questions
Q1: What are case study questions for CBSE examinations?
A1: Case study questions in CBSE examinations typically involve scenarios or real-life examples, requiring students to apply their understanding of concepts to solve problems or analyze situations.
Q2: Why are case study questions important for understanding class 9 science chapters?
A2: Case study questions provide a practical context for students to apply theoretical knowledge to real-world situations, fostering deeper understanding and critical thinking skills.
Q3: How should students approach answering case study questions for CBSE?
A3: Students should carefully read the case study, identify the key issues or problems presented, analyze the information provided, apply relevant concepts and principles of chemical reactions and equations, and formulate well-supported solutions or responses.
Q4: Are there any resources available online for students to practice case study questions on class 9 science chapters for CBSE exams?
A4: Yes, several educational websites offer case study questions for CBSE students preparing for science examinations. We also offer a collection of case study questions for all classes and subject on our website. Visit our website to access these questions and enhance your learning experience. If you need more case study questions for your preparation, then you visit Physics Gurukul website.
Q5: How can students effectively prepare for case study questions on “Is Matter Around Us Pure” for CBSE exams?
A5: Effective preparation strategies include regular revision of concepts, solving practice questions, analyzing case studies from previous exams, seeking clarification on doubts, and consulting with teachers or peers for guidance and support.
Q6: How can teachers incorporate case study questions on “Is Matter Around Us Pure” class 9 science into classroom teaching?
A6: Teachers can integrate case studies into lesson plans, group discussions, or interactive activities to engage students in active learning, promote problem-solving skills, and facilitate a deeper understanding of “Is Matter Around Us Pure”.
Q7: What is meant by a substance?
A7: A material that consists of a single type of particles is known as pure substance. All constituent particles of pure substance have the same chemical nature.
Q8: List the points of differences between homogeneous and heterogeneous mixtures
A8: A homogeneous mixture is a mixture having a uniform composition throughout the mixture.
For example: Salt in water, sugar in water, copper sulphate in water.
A heterogeneous mixture is a mixture having a non-uniform composition throughout the mixture.
For example: Sodium chloride and iron fillings, salt and sulphur, oil and water.
Q9: How can we check the purity of a substance?
A9: The purity of a substance can be checked by its melting point and boiling point. A pure substance has a fixed
melting point or boiling point at constant pressure.
Q10: Define element.
A10: An element is a basic form of matter that cannot be broken down into simpler substances by chemical reaction. An
element consists of only one kind of atom.
Q11: Define compound.
A11: A compound is a substance made up of two or more elements chemically combined with one another. A compound always contains a definite proportion of the elements by mass.
Q12: Name the three states of matter.
A12: The three states of matter are solid, liquid and gas.
Q13: Name the metal which is liquid at room temperature.
A13: Mercury
Q14: How will you separate a mixture of mercury, kerosene and water?
A14: The mixture of mercury, kerosene and water is taken in a separating funnel. Separating funnel is used to separate
two immiscible liquids. The principle is that immiscible liquids separate out in layers depending on their densities. Mercury being the heaviest from the bottom layer, water form the middle layer and kerosene form the top layer. On opening the tap, mercury will run out first, followed by water and kerosene at the end
Download Customised White Label Study Materials in MS Word Format
We are providing teaching resources to teachers and coaching institute looking for customised study materials in MS word format. Our High-quality editable study material which is prepared by the expert faculties are Highly useful for Teachers, Mentors, Tutors, Faculties, Coaching Institutes, Coaching Experts, Tuition Centers.



