Last Updated on April 23, 2025 by XAM CONTENT
Hello students, we are providing case study questions for class 12 chemistry. Case study questions are the new question format that is introduced in CBSE board. The resources for case study questions are very less. So, to help students we have created chapterwise case study questions for class 12 chemistry. In this article, you will find case study questions for cbse class 12 chemistry chapter 1 Solutions.
| Chapter | Solutions |
| Type of Questions | Case Study Questions |
| Nature of Questions | Competency Based Questions |
| Board | CBSE |
| Class | 12 |
| Subject | Chemistry |
| Unit | Unit 2 Solutions |
| Useful for | Class 12 Studying Students |
| Answers provided | Yes |
| Difficulty level | Mentioned |
| Important Link | Class 12 Chemistry Chapterwise Case Study |
Case Study Questions on Solutions
Questions
Question 1:
At 298 K, the vapour pressure of pure benzene, C6H6 is 0.256 bar and the vapour pressure of pure toluene C6H5CH3 is 0.0925 bar. Two mixtures were prepared as follows:
(I) 7.8 g of C6H6 + 9.2 g of toluene
(II) 3.9 g of C6H6 + 13.8 g of toluene
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The total vapour pressure (bar) of solution I is
(a) 0.128
(b) 0.174
(c) 0.198
(d) 0.258
(ii) Which of the given solutions have higher vapour pressure?
(a) I
(b) II
(c) Both have equal vapour pressure
(d) Cannot be predicted
(iii) Mole fraction of benzene in vapour phase in solution I is
(a) 0.128
(b) 0.174
(c) 0.734
(d) 0.266
(iv) Which of the following statements is/are correct?
(I) Mole fraction of toluene in vapour phase is more in solution I.
(II) Mole fraction of toluene in vapour phase is less in solution I.
(III) Mole fraction of benzene in vapour phase is less in solution I.
(a) Only II
(b) Only I
(c) I and III
(d) II and III
Answers
(i) b
(ii) a
(iii) c
(iv) a
Question 2:
Few colligative properties are:
(a) relative lowering of vapour pressure: depends only on molar concentration of solute (mole fraction) and independent of its nature.
(b) depression in freezing point: it is proportional to the molal concentration of solution.
(c) elevation of boiling point: it is proportional to the molal concentration of solute.
(d) osmotic pressure : it is proportional to the molar concentration of solute.
A solution of glucose is prepared with 0.052 g at glucose in 80.2 g of water. (Kf = 1.86 K kg mol–1 and Kb = 5.2 K kg mol–1)
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Molality of the given solution is
(a) 0.0052 m
(b) 0.0036 m
(c) 0.0006 m
(d) 1.29 m
(ii) Boiling point for the solution will be
(a) 373.05 K
(b) 373.15 K
(c) 373.02 K
(d) 372.98 K
(iii) The depression in freezing point of solution will be
(a) 0.0187 K
(b) 0.035 K
(c) 0.082 K
(d) 0.067 K
(iv) Mole fraction of glucose in the given solution is
(a) 6.28 × 10–5
(b) 1.23 × 10–4
(c) 0.00625
(d) 0.00028
Answers
(i) (b): $m=\frac{0.052}{180} \times \frac{1000}{80.2}=0.0036$
(ii) (c): $\Delta T_b=K_b \times m=5.2 \times 0.0036=0.0187 \mathrm{~K}$
$T_b=373+0.0187=373.0187 \mathrm{~K} \approx 373.02 \mathrm{~K}$
(iii) (d): $\Delta T_f=K_f \times m=1.86 \times 0.0036=0.067 \mathrm{~K}$
(iv) (a): Moles of glucose $=\frac{0.052}{180}=0.00028$
Moles of water $=\frac{80.2}{18}=4.455$
Mole fraction of glucose $=\frac{0.00028}{4.45+0.00028}=6.28 \times 10^{-5}$
Also check
Case study questions for other chapters of class 12 chemistry is given below.
We hope the given case study questions for Solutions Class 12 helps you in your learning.
Topics from which case study questions may be asked
- Types of solutions
- Expression of concentration of solutions of solids in liquids
- Solubility of gases in liquids
- Solid solutions
- Raoult’s law
- Colligative properties:
Relative lowering of vapour pressure
Elevation of boiling point
Depression of freezing point
Osmotic pressure - Determination of molecular masses using colligative properties
- Abnormal molecular mass
- Van’t Hoff factor
Aquatic species are more comfortable in cold water rather than in warm water due to presence of more dissolved oxygen.
For further practice on case study questions related to Solutions Class 12 Chemistry, we recommend exploring the link given below.
Quiz
| Topic | Solutions |
| Useful for | CBSE Class 12 Students |
| Type of Questions | MCQs, Case Study, Assertion Reason |
| No. of Questions | 35 |
| Solutions | Instant Solutions after Completion of Quiz |
| Quiz Link | Click here to Take Test |
| Price | Free |
How to take quiz or test using the given link
It’s quite simple!
Step 1: Click on the given link. You will see the below screen.
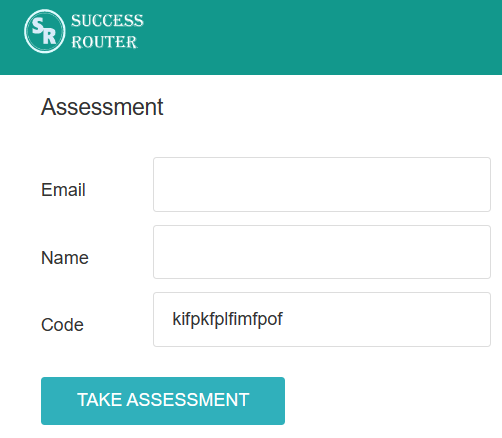
Step 2: Fill in the necessary details. There is no need to register. Just fill your email and name and click on the button “Take Assessment”. The below screen will appear.
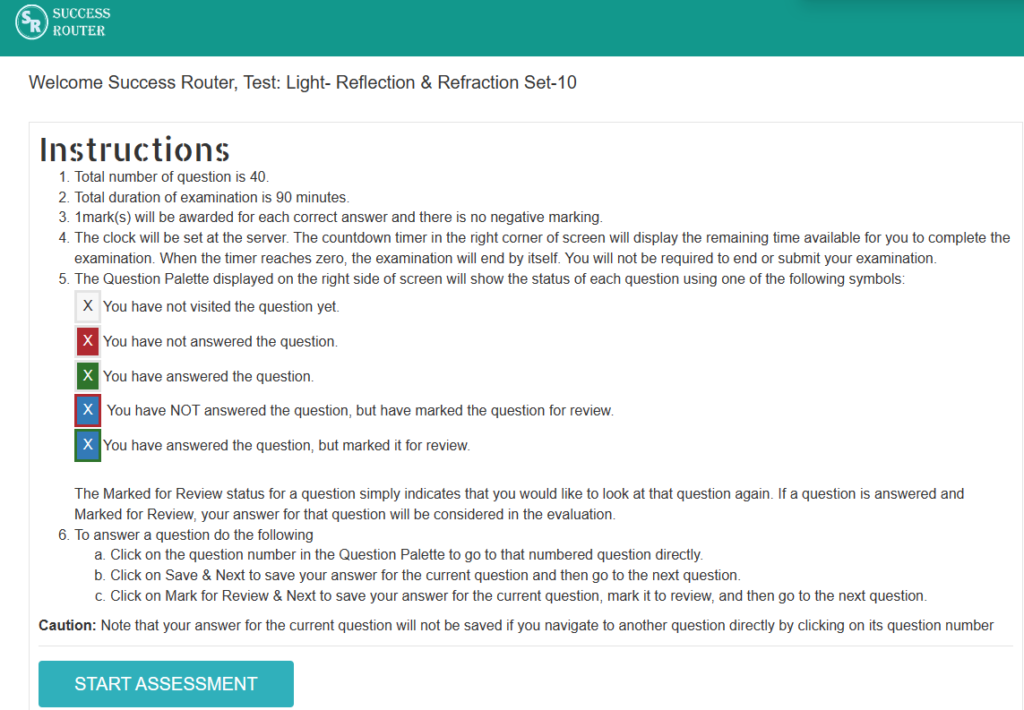
Step 3: Click on start assessment. Now you are ready to take test.
Frequently Asked Questions (FAQs) on Solutions Case Study Questions
Q1: What are case study questions for CBSE examinations?
A1: Case study questions in CBSE examinations typically involve scenarios or real-life examples, requiring students to apply their understanding of concepts to solve problems or analyze situations.
Q2: Why are case study questions important for understanding class 12 chemistry chapters?
A2: Case study questions provide a practical context for students to apply theoretical knowledge to real-world situations, fostering deeper understanding and critical thinking skills.
Q3: How should students approach answering case study questions for CBSE?
A3: Students should carefully read the case study, identify the key issues or problems presented, analyze the information provided, apply relevant concepts and principles of chemical reactions and equations, and formulate well-supported solutions or responses.
Q4: Are there any resources available online for students to practice case study questions on class 12 chemistry chapters for CBSE exams?
A4: Yes, several educational websites offer case study questions for CBSE students preparing for chemistry examinations. We also offer a collection of case study questions for all classes and subject on our website. Visit our website to access these questions and enhance your learning experience. If you need more case study questions for your preparation, then you visit Physics Gurukul website.
Q5: How can students effectively prepare for case study questions on “Solutions” for CBSE exams?
A5: Effective preparation strategies include regular revision of concepts, solving practice questions, analyzing case studies from previous exams, seeking clarification on doubts, and consulting with teachers or peers for guidance and support.
Q6: How can teachers incorporate case study questions on “Solutions” class 12 chemistry into classroom teaching?
A6: Teachers can integrate case studies into lesson plans, group discussions, or interactive activities to engage students in active learning, promote problem-solving skills, and facilitate a deeper understanding of “Solutions”.
Q7: Why is the vapour pressure of an aqueous solution of glucose lower than that of water?
A7: Evaporation is a surface process. Availability of more surface area is responsible for more vaporisation of water. In pure water, complete liquid surface is covered with water molecules. When a non-volatile solute like glucose is dissolved in water, the fraction occupied by solvent molecules at the solvent surface decreases because some place (fraction) is occupied by glucose molecules. Consequently, the number of solvent molecules leaving the surface reduces and hence, vapour pressure of aqueous solution of glucose decreases.
Q8: Explain gram-molecular depression constant.
A8: The depression in freezing point, when 1 gram-molecule of a non-volatile and non-electrolyte substance is dissolved in 100 gram of solvent, is called the gram-molecular or molar depression constant.
It is represented as K100 and its unit is Kmol-1 100 g
Q9: What is semipermeable membrane?
A9: Semipermeable Membranes (SPM) are natural or synthetic continuous sheets or films which contain a network of submicroscopic holes or pores. Parchment paper, egg shell membrane, copper ferrocyanide are semipermeable membranes.
Q10: At low pressure and high temperature, water evaporates rapidly, why?
A10: At low pressure and high temperature, the kinetic energy of water molecules increases. Due to this, more molecules leave the water surface and gets converted into vapour phase. That’s why, evaporation of water occurs rapidly.
Q11: What is azeotrope?
A11: Azeotrope is a binary mixture having the same composition in liquid and vapour phase and boil at a constant temperature.
Q12: What type of deviation from Raoult’s law is shown by a mixture of ethanol and acetone? Give reason.
A12: A mixture of ethanol and acetone shows positive deviation from Raoult’s law. In pure ethanol, molecules are hydrogen bonded and when acetone is added to it, the molecules get in between the most molecules and break some of the hydrogen bonds between them. Due to weakening of interactions, the mixture shows positive deviation from Raoult’s law.
Download Customised White Label Study Materials in MS Word Format
We are providing teaching resources to teachers and coaching institute looking for customised study materials in MS word format. Our High-quality editable study material which is prepared by the expert faculties are Highly useful for Teachers, Mentors, Tutors, Faculties, Coaching Institutes, Coaching Experts, Tuition Centers.



