Last Updated on June 16, 2025 by XAM CONTENT
Mastering the important concepts in Class 11 Chemistry Chapter 1 – Some Basic Concepts of Chemistry is essential for scoring high marks in your exams. That’s why we’ve put together chapterwise multiple-choice questions (MCQs) with answers to help you revise effectively and boost your confidence. It is a part of MCQ Questions for CBSE Class 11 Chemistry Series.
These multiple-choice questions will help you assess your knowledge, improve accuracy, and boost confidence for your exams. Whether you are preparing for school tests, online tests or competitive exams, these Some Basic Concepts of Chemistry MCQs will strengthen your conceptual clarity.
| Chapter | Some Basic Concepts of Chemistry |
| Book | Chemistry for Class 11 |
| Type of Questions | MCQ Questions |
| Nature of Questions | Competency Based Questions |
| Board | CBSE |
| Class | 11 |
| Subject | Chemistry |
| Useful for | Class 11 Studying Students |
| Answers provided | Yes |
| Difficulty level | Mentioned |
| Important Link | Class 11 Chemistry Chapterwise MCQ Questions |
MCQ Questions on Some Basic Concepts of Chemistry Class 11 Chemistry (PDF Download)
MCQs
Q1. A 0.5 mol sample of an ideal gas at 300 K is allowed to expand isothermally from 5.0 L to 10.0 L. What is the work done by the gas? (R = 8.314 J/mol·K)
(a) 432 J
(b) 864 J
(c) 1037 J
(d) 1245 J
Show Answer
Answer: (c)
Explanation: Work = nRT ln(Vf/Vi) = 0.5 × 8.314 × 300 × ln(10/5) = 1037 J.
Q2. Which of the following best describes the limiting reagent in a chemical reaction?
(a) Reactant that remains unused
(b) Reactant that is present in minimum quantity
(c) Reactant that is completely consumed
(d) Reactant that has the lowest molar mass
Show Answer
Answer: (c)
Explanation: The limiting reagent is completely used up in the reaction, thus limiting the amount of product formed.
Q3. What is the empirical formula of a compound containing 40% carbon, 6.7% hydrogen, and 53.3% oxygen by mass?
(a) CH2O
(b) C2H4O2
(c) CHO
(d) C2H6O
Show Answer
Answer: (a)
Explanation: Convert to moles: C = 3.33, H = 6.67, O = 3.33; Divide by smallest: C = 1, H = 2, O = 1 → CH2O.
Q4. Which of the following postulates of Dalton’s atomic theory is no longer valid according to modern atomic theory?
(a) Atoms of the same element are identical in all respects
(b) Atoms can neither be created nor destroyed
(c) Atoms combine in simple whole number ratios
(d) Chemical reactions involve rearrangement of atoms
Show Answer
Answer: (a)
Explanation: Modern theory recognizes isotopes — atoms of the same element with different masses — invalidating this postulate.
Q5. Which law is obeyed when magnesium burns in air to form magnesium oxide without any change in mass?
(a) Law of definite proportions
(b) Law of conservation of mass
(c) Law of multiple proportions
(d) Avogadro’s law
Show Answer
Answer: (b)
Explanation: The total mass of reactants and products remains constant, obeying the law of conservation of mass.
Q6. How many atoms are present in 11 g of carbon dioxide (CO2)?
(a) 3.01 × 1023
(b) 6.02 × 1023
(c) 9.03 × 1023
(d) 1.20 × 1024
Show Answer
Answer: (c)
Explanation: Moles of CO2 = 11/44 = 0.25; Atoms = 0.25 × 6.022×1023 × 3 = 9.03×1023.
Q7. If 4 g of hydrogen reacts with 32 g of oxygen to form water, how much water is formed?
(a) 18 g
(b) 36 g
(c) 34 g
(d) 30 g
Show Answer
Answer: (b)
Explanation: H2 + 1/2 O2 → H2O; total mass = 4 + 32 = 36 g of water.
Q8. Which of the following expresses molarity?
(a) mol/kg
(b) mol/L
(c) g/mol
(d) mol/m3
Show Answer
Answer: (b)
Explanation: Molarity is moles of solute per liter of solution, expressed in mol/L.
Q9. What is the volume at STP occupied by 2 moles of an ideal gas?
(a) 11.2 L
(b) 22.4 L
(c) 33.6 L
(d) 44.8 L
Show Answer
Answer: (d)
Explanation: At STP, 1 mole of ideal gas occupies 22.4 L; so, 2 moles occupy 44.8 L.
Q10. Which of the following pairs illustrates the law of multiple proportions?
(a) CO and CO2
(b) NaCl and KCl
(c) HCl and H2SO4
(d) CH4 and C2H6
Show Answer
Answer: (a)
Explanation: CO and CO2 show different masses of oxygen combining with a fixed mass of carbon, exemplifying the law.
Q11. Avogadro’s law implies that equal volumes of gases at the same temperature and pressure have:
(a) Equal masses
(b) Equal densities
(c) Equal numbers of molecules
(d) Equal molecular masses
Show Answer
Answer: (c)
Explanation: Avogadro’s law states equal volumes of all gases under identical conditions contain equal number of molecules.
Q12. One mole of calcium phosphate, Ca3(PO4)2, contains how many moles of calcium ions?
(a) 1
(b) 2
(c) 3
(d) 6
Show Answer
Answer: (c)
Explanation: Each formula unit contains 3 Ca2+ ions, hence 1 mole gives 3 moles of calcium ions.
Q13. The number of moles in 4.9 g of H2SO4 is:
(a) 0.05 mol
(b) 0.10 mol
(c) 0.25 mol
(d) 0.50 mol
Show Answer
Answer: (b)
Explanation: Molar mass of H2SO4 = 98 g/mol; Moles = 4.9/98 = 0.05 mol.
Q14. A sample of gas occupies 400 mL at 300 K. What will be its volume at 600 K at constant pressure?
(a) 200 mL
(b) 400 mL
(c) 800 mL
(d) 1000 mL
Show Answer
Answer: (c)
Explanation: V1/T1 = V2/T2; V2 = 400 × 600 / 300 = 800 mL.
Q15. The SI unit of amount of substance is:
(a) Mole
(b) Gram
(c) Kilogram
(d) Liter
Show Answer
Answer: (a)
Explanation: The mole is the SI base unit for the amount of substance, defined by the number of atoms in 12 g of C-12.
Q16. The mass of 1 atom of carbon-12 is:
(a) 12 g
(b) 1.99 × 10-23 g
(c) 1.66 × 10-24 g
(d) 1.99 × 10-26 kg
Show Answer
Answer: (d)
Explanation: Mass of 1 C-12 atom = 12 g / 6.022 × 1023 = 1.99 × 10-26 kg.
Q17. Which physical quantity is measured in kg·m2/s2?
(a) Pressure
(b) Work
(c) Force
(d) Power
Show Answer
Answer: (b)
Explanation: The SI unit kg·m2/s2 corresponds to energy or work.
Q18. Which of the following quantities is not a state function?
(a) Internal energy
(b) Enthalpy
(c) Work
(d) Temperature
Show Answer
Answer: (c)
Explanation: Work depends on the path taken during a process, unlike state functions which depend only on the current state.
Q19. Which of the following statements is true for a chemical reaction?
(a) Mass and energy are destroyed
(b) Mass and energy are conserved
(c) Only mass is conserved
(d) Only energy is conserved
Show Answer
Answer: (b)
Explanation: According to the laws of conservation, both mass and energy remain conserved in a chemical process.
Q20. Which instrument is used to determine the mass of an atom accurately?
(a) Manometer
(b) Barometer
(c) Mass spectrometer
(d) Thermometer
Show Answer
Answer: (c)
Explanation: A mass spectrometer accurately measures the masses of atoms and isotopic composition.
We hope the given mcq questions with Answers for Some Basic Concepts of Chemistry Class 11 helps you in your learning.
Topicwise MCQs Coverage
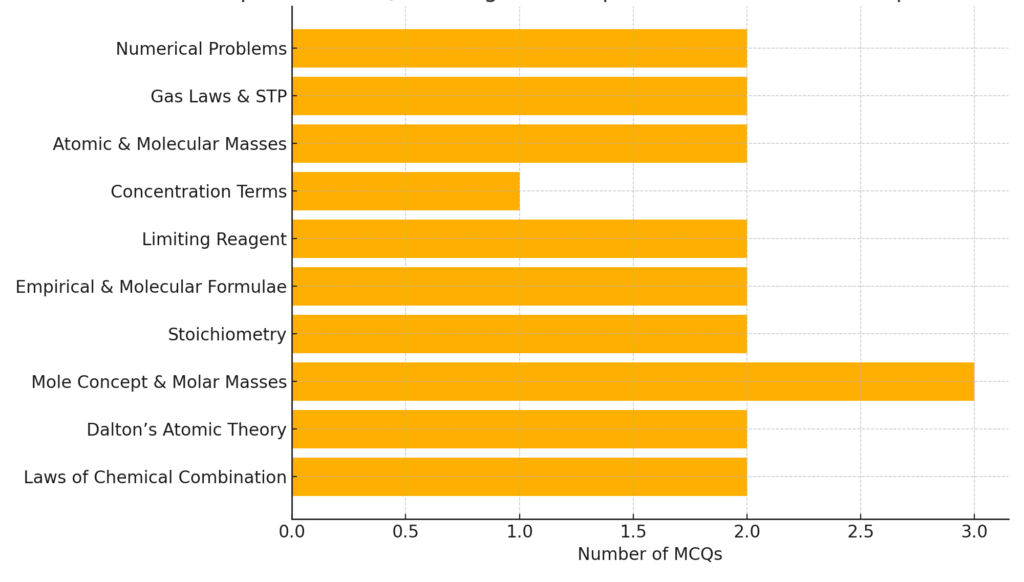
Also check
- Structure of Atom – Class 11 Chemistry Chapter 2 MCQ Questions with Answers (Updated)
- Some Basic Concepts of Chemistry – Class 11 Chemistry Chapter 1 MCQ Questions with Answers (Updated)
🚀 Boost Your Exam Prep: Get MCQ Questions for all subjects (Class 6-12) now!
👉 Explore more resources on CBSE Class 11
Topics from which mcq questions may be asked
- Mole concept
- Stoichiometry
- Atomic and molecular masses
The journey of chemistry begins with the mole and measurements.
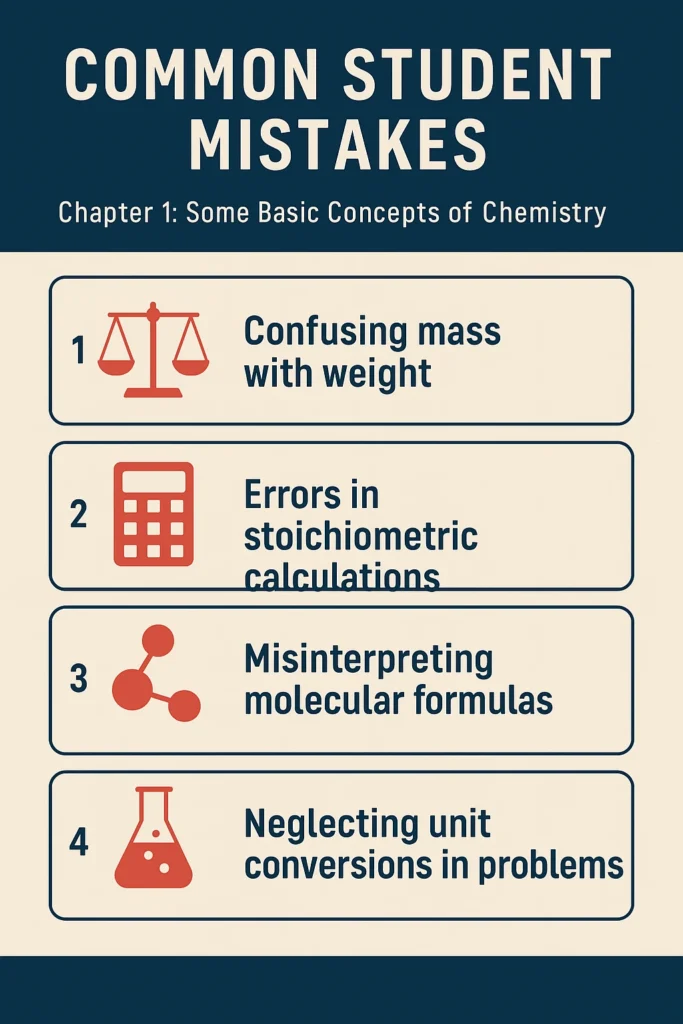
Common Student Mistakes
Frequently Asked Questions (FAQs) on Some Basic Concepts of Chemistry MCQ Questions
Q1: What type of MCQs are included for Class 11 Chemistry?
A1: The MCQs cover conceptual, numerical, application-based, and competency-based questions from all chapters of the NCERT Class 11 Chemistry book, following the latest CBSE guidelines.
Q2: Are these MCQs suitable for NEET or competitive exams?
A2: Yes, many of the MCQs are designed with a higher level of difficulty, suitable for NEET, JEE, and other entrance exams, especially in the Organic and Physical Chemistry sections.
Q3: Do these Class 11 Chemistry MCQs follow the updated CBSE syllabus?
A3: Absolutely. All questions are aligned with the latest CBSE syllabus and NCERT textbook for Class 11 Chemistry.
Q4: Are answers and explanations provided with each MCQ?
A4: Yes. Each MCQ comes with the correct answer and, wherever needed, a short explanation to help students understand the concept better.
Q5: How should I use these MCQs for exam preparation?
A5: Practice chapterwise first, then take topic-based timed quizzes. Focus on tricky and frequently asked concepts. Revise wrong answers and refer to the explanation provided.
Q6: Can teachers and coaching institutes use this material?
A6: Definitely! These MCQs are ideal for classroom assignments, weekly tests, online quizzes, and evaluation by teachers, schools, and coaching centers.
Q7: Where can I find chapterwise Class 11 Chemistry MCQ questions with answers?
A7: You can find chapterwise MCQ questions with answers and detailed explanations on trusted educational platforms like xamcontent.com and physicsgurukul.com. These cover both basic and advanced-level questions.



