Last Updated on November 16, 2025 by XAM CONTENT
Hello students, we are providing case study questions for class 12 physics. Case study questions are the new question format that is introduced in CBSE board. The resources for case study questions are very less. So, to help students we have created chapterwise case study questions for class 12 physics. In this article, you will find case study questions for cbse class 12 physics chapter 12 Atoms.
| Chapter | Atoms |
| Type of Questions | Case Study Questions |
| Nature of Questions | Competency Based Questions |
| Board | CBSE |
| Class | 12 |
| Subject | Physics |
| Unit | Unit 8 Atoms and Nuclei |
| Useful for | Class 12 Studying Students |
| Answers provided | Yes |
| Difficulty level | Mentioned |
| Important Link | Class 12 Physics Chapterwise Case Study |
Case Study Questions on Atoms
Questions
Question 1:
Hydrogen is the simplest atom of nature. There is one proton in its nucleus and an electron moves around the nucleus in a circular orbit. According to Niels Bohr, this electron moves in a stationary orbit. When this electron is in the stationary orbit, it emits no electromagnetic radiation. The angular momentum of the electron is quantized, i.e., $m v r=(n h / 2 \pi)$, where $m=$ mass of the electron, $v=$ velocity of the electron in the orbit, $r=$ radius of the orbit and $n=1,2,3, \ldots$. When transition takes place from $k$ th orbit to $j$ th orbit, energy photon is emitted. If the wavelength of the emitted photon is $\lambda$, we find that $\frac{1}{\lambda}=R\left[\frac{1}{j^2}-\frac{1}{k^2}\right]$, where $R$ is Rydberg’s constant.
On a different planet, the hydrogen atom’s structure was somewhat different from ours. The angular momentum of electron was $P=2 n(h / 2 \pi)$, i.e., an even multiple of $(h / 2 \pi)$.
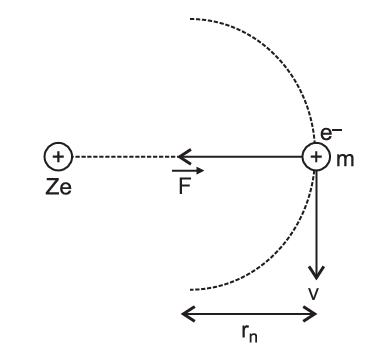
Read the given passage carefully and give the answer of the following questions:
Q. 1. What is the minimum permissible radius of the orbit?
Q. 2. In our world, the velocity of electron is v0, when the hydrogen atom is in the ground state. Find the velocity of electron in this state on the other planet.
Q. 3. In our world, the ionisation potential energy of a hydrogen atom is 13.6 eV. What will be the ionisation potential energy on the other planet?
Q. 4. What is the total energy (En) of the electron in the stationary states in the nth orbit of the hydrogen atom?
Answers
1. On other planet, $m v r=2 n \frac{h}{2 \pi} \Rightarrow v=\frac{n h}{\pi m r}$
$$
\Rightarrow \quad \frac{m v^2}{r}=\frac{1}{4 \pi \varepsilon_0} \frac{e^2}{r^2} \Rightarrow \frac{m n^2 h^2}{\pi^2 m^2 r^3}=\frac{1}{4 \pi \varepsilon_0} \frac{e^2}{r^2}
$$
Putting $n=1$, we get $r=\frac{4 h^2 \varepsilon_0}{m \pi e^2}$
2. On our planet, $v_0=\frac{e^2}{2 \varepsilon_0 n h}$
On other planet, $v=\frac{e^2}{2 \varepsilon_0(2 n) h}=\frac{v_0}{2}$
3. On our planet, $E_n=-\frac{13.6}{n^2}$
On other planet, $E_n^{\prime}=-\frac{13.6}{(2 n)^2}$
$\Rightarrow E_n^{\prime}=\frac{E_n}{4}=\frac{13.6}{4}=3.4 \mathrm{eV}$
4. $\frac{-13.6}{n^2} \mathrm{eV}$
Also check
Case study questions for other chapters of class 12 physics is given below.
- Semiconductor Electronics Class 12 Case Study Questions Physics Chapter 14
- Nuclei Class 12 Case Study Questions Physics Chapter 13
- Atoms Class 12 Case Study Questions Physics Chapter 12
- Dual Nature of Radiation and Matter Class 12 Case Study Questions Physics Chapter 11
- Wave Optics Class 12 Case Study Questions Physics Chapter 10
- Ray Optics and Optical Instruments Class 12 Case Study Questions Physics Chapter 9
- Electromagnetic Waves Class 12 Case Study Questions Physics Chapter 8
- Alternating Current Class 12 Case Study Questions Physics Chapter 7
- Electromagnetic Induction Class 12 Case Study Questions Physics Chapter 6
- Magnetism and Matter Class 12 Case Study Questions Physics Chapter 5
- Moving Charges and Magnetism Class 12 Case Study Questions Physics Chapter 4
- Current Electricity Class 12 Case Study Questions Physics Chapter 3
- Electrostatic Potential and Capacitance Class 12 Case Study Questions Physics Chapter 2
- Electric Charges and Fields Class 12 Case Study Questions Physics Chapter 1
- Case Study Questions for Class 12 Physics
We hope the given case study questions for Atoms Class 12 helps you in your learning.
Download eBooks for CBSE Class 12 Physics (Exam Special)
- 120 Case Study Questions for Class 12 Physics
- 320 Assertion Reasoning Questions for Class 12 Physics
- 95 Reasoning Based Questions for Class 12 Physics
- Important Derivation for Class 12 Physics
- MCQ Questions for Class 12 Physics
- Chapter Test for Class 12 Physics with Solutions
- 230 Important Numerical for Class 12 Physics
- 75 Important Diagrams & Graphs for Class 12 Physics
Topics from which case study questions may be asked
- Alpha-Particle Scattering Experiment
- Rutherford’s Model of Atom
- Bohr Model of Hydrogen Atom
- Expression for Radius of nth Orbit
- Velocity and Energy of Electron in nth Orbit
- Hydrogen Line Spectra (Qualitative Treatment Only)
Two electric field lines never cross each other. If they intersect, then there will be two directions of electric field at the point of intersection which is not possible.
For further practice on case study questions related to Atoms Class 12 Physics, we recommend exploring the link given below.
Frequently Asked Questions (FAQs) on Atoms Case Study Questions
Q1: What are case study questions for CBSE examinations?
A1: Case study questions in CBSE examinations typically involve scenarios or real-life examples, requiring students to apply their understanding of concepts to solve problems or analyze situations.
Q2: Why are case study questions important for understanding class 12 physics chapters?
A2: Case study questions provide a practical context for students to apply theoretical knowledge to real-world situations, fostering deeper understanding and critical thinking skills.
Q3: How should students approach answering case study questions for CBSE?
A3: Students should carefully read the case study, identify the key issues or problems presented, analyze the information provided, apply relevant concepts and principles of chemical reactions and equations, and formulate well-supported solutions or responses.
Q4: Are there any resources available online for students to practice case study questions on class 12 physics chapters for CBSE exams?
A4: Yes, several educational websites offer case study questions for CBSE students preparing for science examinations. We also offer a collection of case study questions for all classes and subject on our website. Visit our website to access these questions and enhance your learning experience. If you need more case study questions for your preparation, then you visit Physics Gurukul website.
Q5: How can students effectively prepare for case study questions on “Atoms” for CBSE exams?
A5: Effective preparation strategies include regular revision of concepts, solving practice questions, analyzing case studies from previous exams, seeking clarification on doubts, and consulting with teachers or peers for guidance and support.
Q6: How can teachers incorporate case study questions on “Atoms” class 12 physics into classroom teaching?
A6: Teachers can integrate case studies into lesson plans, group discussions, or interactive activities to engage students in active learning, promote problem-solving skills, and facilitate a deeper understanding of “Atoms”.
Q7: Name the experiment responsible for the discovery of atomic nucleus.
A7: Rutherford’s a-scattering experiment.
Q8: Define ionisation energy.
A8: It is defined as the energy required to remove an electron from an atom, i.e., the energy required to take an electron from its ground state to the outermost orbit (n = ∞).
Download Customised White Label Study Materials in MS Word Format
We are providing teaching resources to teachers and coaching institute looking for customised study materials in MS word format. Our High-quality editable study material which is prepared by the expert faculties are Highly useful for Teachers, Mentors, Tutors, Faculties, Coaching Institutes, Coaching Experts, Tuition Centers.



