Last Updated on April 2, 2025 by XAM CONTENT
Hello students, we are providing assertion and reason questions for class 10 science. Assertion reason questions are the new question format that is introduced in CBSE board. The resources for assertion reason questions are very less. So, to help students we have created chapterwise assertion reason questions for class 10 science. In this article, you will find assertion reason questions for CBSE Class 10 Science Chapter 10 Human Eye and Colourful World.
| Chapter | Chemical Reactions and Equations |
| Type of Questions | Assertion Reason Questions |
| Nature of Questions | Competency Based Questions |
| Board | CBSE |
| Class | 10 |
| Subject | Science |
| Unit | Unit 1: Chemical substances – Nature and Behaviour |
| Useful for | Class 10 Studying Students |
| Answers provided | Yes |
| Difficulty level | Mentioned |
| Important Link | Class 10 Science Chapterwise Assertion Reason |
Assertion Reason Questions on Chemical Reactions and Equations
Directions:
(a) Both A and R are true and R is correct explanation of the assertion.
(b) Both A and R are true but R is not the correct explanation of the assertion.
(c) A is true but R is false.
(d) A is false but R is true.
Q. 1. Assertion (A): Rusting of iron is endothermic in nature.
Reason (R): As the reaction is slow, the release of heat is barely evident.
(CBSE SQP 2023-24)
Difficulty Level: Medium
Ans. (d) Assertion (A) is false but Reason (R) is true. Assertion (A) is false because rusting of iron is an exothermic reaction.
Q. 2. Assertion (A): Burning of natural gas is an endothermic process.
Reason (R): Methane gas combines with oxygen to produce carbon dioxide and water.
(CBSE 2021 Term-1)
Difficulty Level: Medium
Ans. (d) Assertion (A) is false but Reason (R) is true. Assertion is false because burning of natural gas is an exothermic reaction.
Q. 3. Assertion (A): Reaction of quicklime with water is an exothermic reaction.
Reason (R): Quicklime reacts vigorously with water releasing a large amount of heat.
(CBSE 2023)
Difficulty Level: Medium
Ans. (a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
Q. 4. Assertion (A): When an iron rod is dipped into a solution of copper sulphate, copper is displaced.
Reason (R): Iron is more reactive than copper.
Difficulty Level: Medium
Ans. (a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
Q. 5. Assertion (A): Silver bromide decomposition is used in black and white photography.
Reason (R): Light provides energy for this exothermic reaction.
(CBSE SQP 2022-23)
Difficulty Level: Medium
Ans. (c) Assertion (A) is true but Reason (R) is false. Reason (R) is false because light provides energy for photochemical decomposition reaction.
Also check
- Chemical Reactions and Equations Class 10 Assertion Reason Questions Science Chapter 1
- Human Eye and Colourful World Class 10 Assertion Reason Questions Science Chapter 10
- Life Processes Class 10 Assertion Reason Questions Science Chapter 6
- Light – Reflection and Refraction Class 10 Assertion Reason Questions Science Chapter 10
You may also like
Download eBooks for CBSE Class 10 Science Chemical Reactions and Equations
- Chemical Reactions and Equations Important Questions for CBSE Class 10 Science
- Chemical Reactions and Equations Chapter Test
Helpful Links for CBSE Class 10 Science Preparation
- Download 125 Important Case Study Questions for CBSE Class 10 Science
- Download 220 Important Assertion Reason Questions for CBSE Class 10 Science
- Download 225 Practical Based Questions for CBSE Class 10 Science
- Download 65 Important Numerical Problems for CBSE Class 10 Physics
- Download 60 Important Diagram Based Questions for CBSE Class 10 Physics
- Download 150 Most Repeated Questions for CBSE Class 10 Science
- Download Chapter Test for CBSE Class 10 Science
Topics from which assertion reason questions may be asked
- Chemical reactions
- Balanced chemical equation
- Types of chemical reactions
- Oxidation and reduction
You are going to study the above topics in class 10 Chemical Reactions and Equations chapter. So, assertion reason questions based on above topics may be asked.
We hope the given Assertion and Reason Questions for Chemical Reactions and Equations Class 10 Science helps you in your learning.
Quiz
| Topic | Chemical Reactions and Equations |
| Useful for | CBSE Class 10 Students |
| Type of Questions | MCQs |
| No. of Questions | 50 |
| Solutions | Instant Solutions after Completion of Quiz |
| Quiz Link | Click here to Take Test |
| Price | Free |
How to take quiz or test using the given link
It’s quite simple!
Step 1: Click on the given link. You will see the below screen.
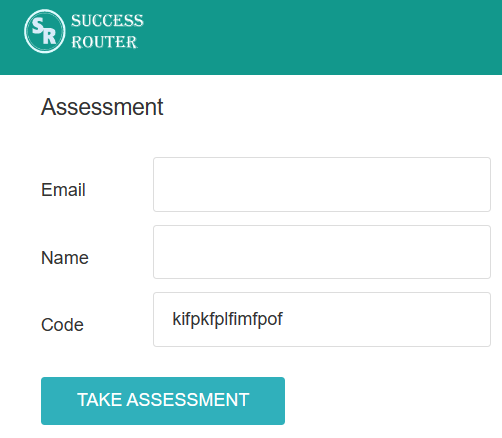
Step 2: Fill in the necessary details. There is no need to register. Just fill your email and name and click on the button “Take Assessment”. The below screen will appear.
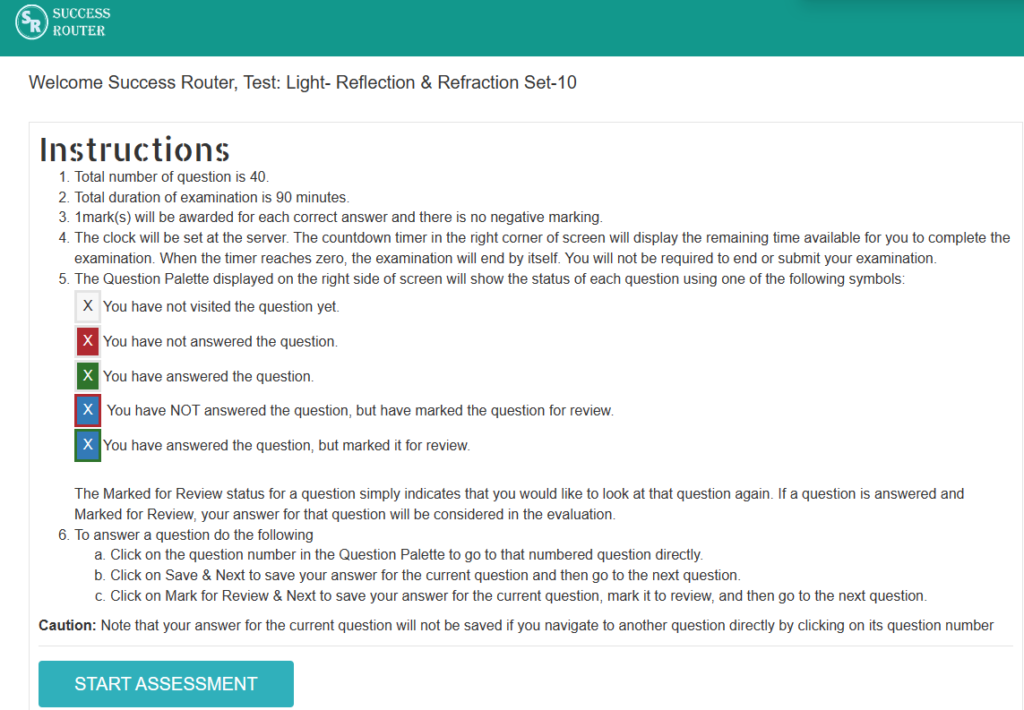
Step 3: Click on start assessment. Now you are ready to take test.

Frequently Asked Questions (FAQs) on Chemical Reactions and Equations Assertion Reason Questions
Q1: What are assertion reason questions?
A1: Assertion-reason questions consist of two statements: an assertion (A) and a reason (R). The task is to determine the correctness of both statements and the relationship between them. The options usually include:
(i) Both A and R are true, and R is the correct explanation of A.
(ii) Both A and R are true, but R is not the correct explanation of A.
(iii) A is true, but R is false.
(iv) A is false, but R is true. or A is false, and R is also false.
Q2: What strategies should students use to answer assertion reason questions effectively?
A2: Students can use the following strategies:
Understand Each Statement Separately: Determine if each statement is true or false independently.
Analyze the Relationship: If both statements are true, check if the reason correctly explains the assertion.
Q3: What are common mistakes to avoid when answering Assertion Reason questions?
A3: Common mistakes include:
Not reading the statements carefully and missing key details.
Assuming the Reason explains the Assertion without checking the logical connection.
Confusing the order or relationship between the statements.
Overthinking and adding information not provided in the question.
Q4: How can practicing assertion reason questions help students?
A4: Practicing assertion-reason questions can help students in several ways:
Improved Conceptual Understanding: It helps students to better understand the concepts by linking assertions with their reasons.
Enhanced Analytical Skills: It enhances analytical skills as students need to critically analyze the statements and their relationships.
Better Exam Preparation: These questions are asked in exams, and practicing them can improve your performance.
Q5: Are there any online resources or tools available for practicing Chemical Reactions and Equations assertion reason questions?
A13: We provide assertion reason questions for CBSE Class 10 Science on our website. Students can visit the website and practice sufficient assertion reason questions and prepare for their exams. If you need more assertion reason questions, then you can visit Physics Gurukul website. they are having a large collection of assertion reason questions for all classes.


